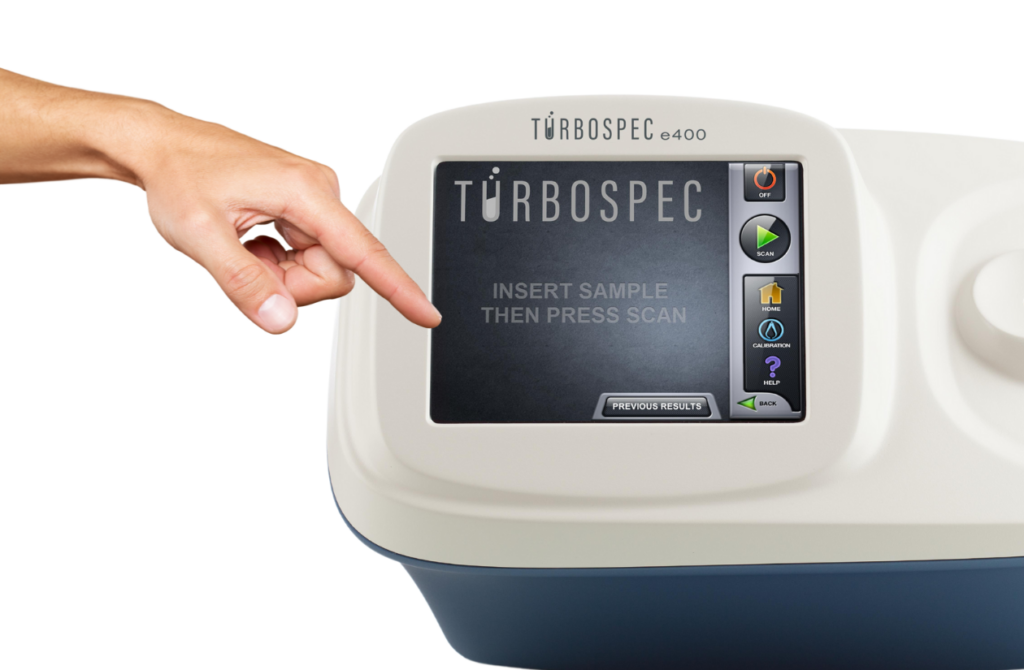
Turbospec LLC uses proprietary quantitative NMR methods to rapidly and accurately determine the concentration of elements in a solution. The base technology is broadly used in clinical MRI and analytical chemistry. In the Turbospec e400, samples are placed in the strong magnetic field of a compact, permanent magnet.
Turbospec e400 Nuclear Magnetic Resonance in Simple Words
Nuclear magnetic resonance (NMR) is defined as an invaluable tool for ascertaining the chemical structures of materials and for gaining insight in to rates of reaction, for example.
In simple terms, an NMR device enables the examination of the molecular composition of a substance by observing and quantifying the interplay of nuclear spins in the presence of a strong magnetic field.
Our proprietary quantitative NMR methods are used to provide fast, accurate and repeatable results.
With Tubrospec’s proprietary quantitative NMR method, samples are placed in a strong magnetic field. NMR signals are then generated as a response to a series of controlled radio-frequency pulses. The signals penetrate the solution to observe selected atoms – therefore samples don’t need to be filtered or diluted.
- This method does not use optical means and therefore it inspects the entire sample, not only the volume that can be reached by light sources. This radically simplifies sample preparation
- The Magnetic Resonance response is independent of the chemical structure; therefore, the signals are comparable to a single calibration standard.
- In contrast to other techniques such as UV-Vis or GC-FID detection the analyst must calibrate the response of the instrument for each unique molecular species, resulting in an inefficient process.
- The Turbospec e400 measures the concentration of specific elements in levels from parts per million to saturation.
Measurement Settings
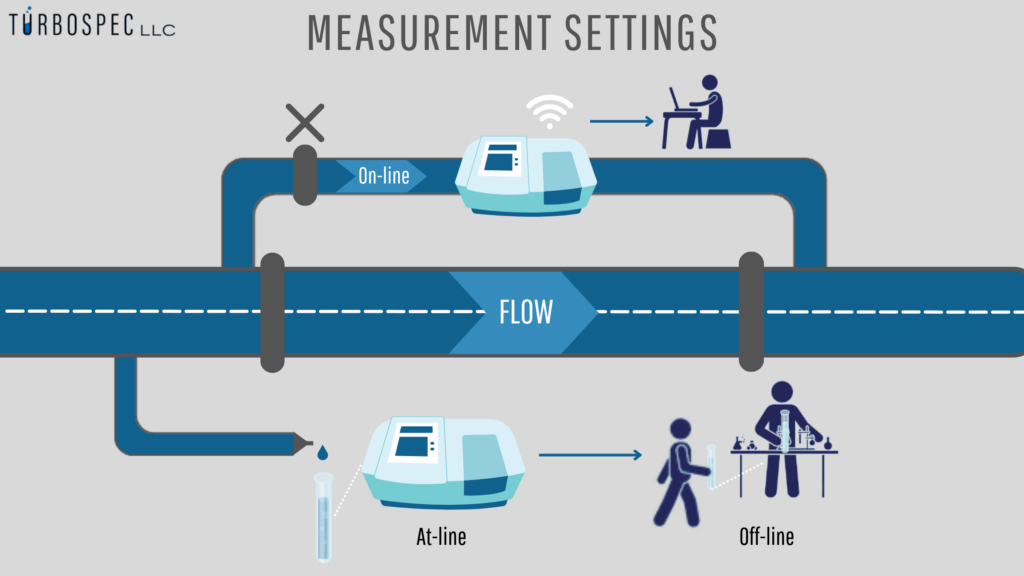
The Turbospec is used in the laboratory (offline) and near lithium production (online). We are currently working to measure directly from the tubes (on-line), as the graph shows. Online and at-line are frequently used remotely – by cable or WiFi.
Turbospec and Magnetic Resonance: How Does It Work?

The Turbospec e400 elemental analyzer makes it easy to determine the concentration of an element in a solution.
Using a robust method and auto-tuned measurements, it precisely calculates the number of atoms per unit of volume.
Thanks to its Magnetic Resonance technology and user-friendly interface, there’s minimal to no need for sample preparation time. Just a quick single-point calibration, and you’re set up in under a minute.
What does this mean for you? Fast and accurate element analysis with reduced wait times, making your processes more efficient.
Counting Atoms
At the core of every atom is the nucleus, consisting of a dense assembly of protons and neutrons.
The number of protons is the atomic number, and determines what element it is; the sum of the number of protons and neutrons is the mass number and determines the particular isotope of the element under consideration.
For example, there are 3 isotopes with a single proton, i.e. hydrogen, deuterium, and tritium; all three respond differently in a magnetic field.
Protons and neutrons are particles that have a quantum mechanical property called “spin” and an associated magnetic moment. When protons and neutrons combine to make a nucleus, their individual spins combine to form a net spin associated with this atom.
The corresponding magnetic moment makes the nucleus act like a small magnet.
In a liquid solution, all those small “magnets” are randomly oriented and therefore the sample has a zero net magnetic moment. If the solution is placed in the field of a permanent magnet, however, nuclei effectively align so that they are pointing in the direction of the external magnetic field generated by the magnet.
This is a state of minimal energy for the spin system.

Randomly oriented nuclei in the absence of a magnetic field – left. Nuclear spins effectively oriented in the direction of the strong magnetic field – right.
If the right amount of energy (radio-frequency in the Magnetic Resonance method) is supplied to the sample, the nuclei can temporarily move from the low energy state.
This means they are out of equilibrium, rotated away from the magnetic field’s direction. In the Turbospec e400, the energy is supplied by applying a burst of oscillating magnetic field whose frequency is tuned precisely to the resonance frequency of the isotope being measured.
The Magnetic Resonance frequencies are similar to those used to broadcast radio signals.

After the radio-frequency field has been applied to tip the nuclei away from their equilibrium orientation, it is switched off rapidly, and the nuclei rotate around the magnetic field produced by the permanent magnet in a process called “precession”.
Eventually, the nuclei re-align with the magnetic field and precession stops. While precession is occurring, the nuclei act like a rotating magnet, which can be detected in an antenna placed around the sample.
The signal detected is much smaller than the radio-frequency pulse that was used to excite the nuclei; therefore, it is highly amplified using low-noise receiver electronics in order to be measured.
The more of those nuclei that are present in the sample, the larger the signal that is detected – signal amplitude is therefore the fundamental measurement made by the Turbospec e400.
The signal decreases with time as the nuclei return to equilibrium. The speed of this decrease depends on the efficiency with which the nuclei can exchange energy among themselves and give up their excess energy to their surroundings.
Measuring the rate of decay of the signal provides further information about the chemical and physical environment of the analyzed nuclei, enabling further characterization of the material and its properties.

A radio-frequency pulse of the right length and frequency can rotate the orientation of the nuclei. Nuclei in the figure on the left are oriented in the direction of the static field. After an RF pulse is applied, the nuclei are rotated – see the figure on the right.
This is the principle that allows the Turbospec e400 to provide accurate measurements of the concentration of certain elements in a solution.
This determination is critical for numerous industrial applications, such as the food and beverages industry and the mining industry, in addition to quality and process control.
Traditional Measurement Methods
Currently, laboratories typically rely upon two standards to measure elemental concentration, such as Sodium in food. The first one is AES, Atomic Emission Spectroscopy, and the second is titration.
Atomic Emission Spectroscopy (AES).
The elemental concentration in most samples is well above the working level of AES, and therefore samples must be diluted to a measurable range.
They may also be subjected to digestion with both an acid and an oxidizer to release bound sodium. The digestion and dilution steps are significant sources of measurement error.
Where AES is subject to interference from other ions including potassium, established methods call for measuring extra samples, which vary the background concentration of these elements in order to determine whether a correction is necessary.
Finally, AES requires the participation of a trained analyst, substantial consumables and regular maintenance.
Titration
A second common methodology is titration for Chloride ions. This is an indirect measurement. The Sodium level is inferred because the primary source of Sodium comes from the addition of Sodium Chloride during the manufacturing process.
A 5-minute procedure is needed to titrate the solution after dilution and sometimes digestion. Although the method itself is rather straightforward, the titration technique uses standardized Silver Nitrate solution, which is expensive, making titration both time-consuming and costly on an on-going basis.
When replacing the titration method, the return on investment for the Turbospec e400 is typically mere months.
In a major innovation for its technical field, the Turbospec e400 ensures that many samples can be measured as-is.
The analyzer significantly improves upon traditional elemental analysis methods, providing single and multi-element testing within a sole instrument. Its rapid, precise analysis capabilities directly meet the needs of a wide range of users.
Contact Us
Turbospec LLC is already helping industries around the world such as the food and mining industries. Contact us today by filling out this form.